The Health Ministry this week abruptly moved out 23 of the National Medicines Regulatory Authority’s (NMRA) most experienced pharmacists, leaving the institution with just 26 mostly junior pharmacists to carry out activities related to drug and safety efficacy.
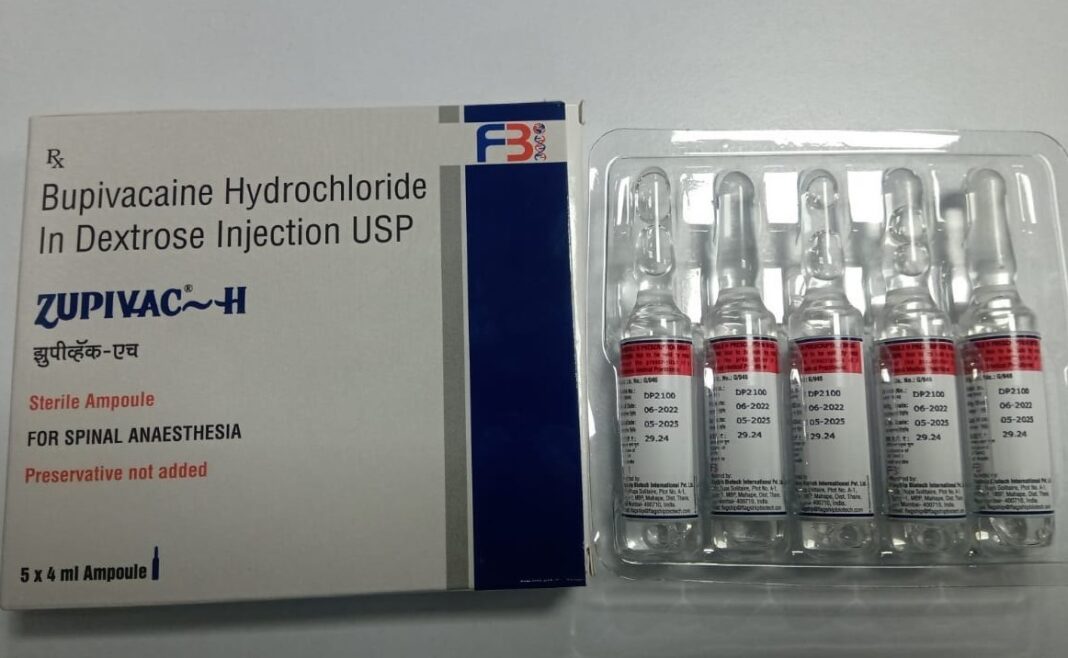
The unexpected transfers of pharmacists come amidst the second reported death on Friday of a patient at the Peradeniya Teaching Hospital after he received the Indian-manufactured “bupivacaine” anaesthetic branded “Zupivac-H”.
The school principal died from complications related to the injection, hospital authorities said. She had been in intensive care since April, when she was administered the anaesthetic in preparation for a hernia operation. The first fatality was a pregnant woman who had also suffered complications.
Zupivac-H, manufactured by Divine Laboratories (Pvt) Ltd in the state of Gujarat, was subsequently withdrawn. Tests found the batch to be substandard. The injection was imported to Sri Lanka on a “waiver of registration” (WoR) issued by the NMRA Chief Executive Officer.
This means Zupivac-H bypassed the stringent vetting process medicines are usually subjected to by the regulator. The NMRA online database shows that it was not registered in Sri Lanka. A senior source from the Society of Government Pharmacists (SoGP) said that WoRs, which are meant to be used as exceptions to the rule, have now become “routine”. “Many drugs are now being issued WoRs by the CEO,” the source said. “Every day, lists are coming.”
On Friday, the SoGP carried out a sick note campaign in protest of the transfers of pharmacists from the NMRA. It was called off yesterday and Health Minister Keheliya Rambukwella has been given seven days to respond. If he fails to do so, the Society will coordinate action with other health sector unions, the source said.
“The transfers had no basis,” he pointed out. “The letter was issued by NMRA Chairman S. B. Jayaratne and said it was being implemented on the instructions of the Secretary of Health. However, when we met the Health Secretary, he denied he had issued transfer orders and that it had been a misinterpretation of his instructions.”
“The Secretary issued a fresh letter instructing the NMRA to reverse the decision and to absorb the pharmacists,” he continued. “The CEO refused to accept it and said the transfers were based on a Board decision. We have not seen such a decision.”
NMRA pharmacists are responsible for medicines evaluation, pharmacovigilance activity and other processes related to the safety of medicine including adverse reactions as well as coordination and licensing. They are the World Health Organisation’s focal point for substandard and falsified medical products. They also contribute towards pricing decisions based on established parameters.
Medical and pharmaceutical industry professionals are now expressing serious concern regarding what they see as a “systematic dismantling of the drugs regulatory apparatus”. The terms of office of members of the NMRA’s Pricing Committee expired recently and were not renewed–thereby leaving pricing decisions also in the hands of its politically-appointed CEO.