Ted Andersen/Geneva.
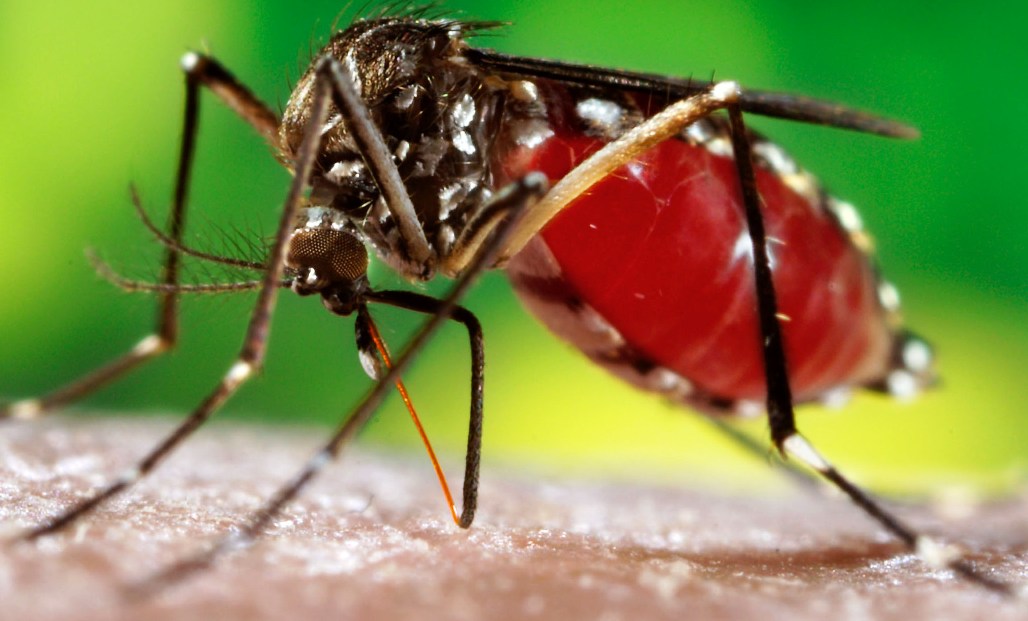
The World Health Organization (WHO) on Friday endorsed the world’s first-ever vaccine for dengue fever, a potentially deadly mosquito-borne virus that threatens to infect close to half of the world’s population.
Unlike malaria, there is no established cure for dengue fever, which can cause severe nausea, bone pain, headaches, rashes, bleeding and even death. The virus can last for up to 10 days. About 390 million people are infected by dengue each year in some 120 countries, particularly in Southeast Asia, Latin America and Africa.
Known as Dengvaxia, the vaccine is the product of two decades of research by French-based Sanofi Pasteur. Four countries—Mexico, Brazil, El Salvador and the Philippines—have already licensed Dengvaxia, but Friday’s recommendation will likely spur a host of other developing nations to follow suit at a time when climate change and urbanization is putting increasing numbers of people at risk from the mosquito-borne disease. “In countries where dengue is endemic, it’s one of the most feared diseases,” says Dr. In-Kyu Yoon, director of the Dengue Vaccine Initiative, an international consortium that has partnered with Sanofi. “The trajectory globally is increasing—at this point it’s essentially a pandemic.”
The vaccine is given in three injections spaced out over one year. It is designed for those over the age of nine who have been previously exposed to the virus and is best suited for people living in endemic areas, as opposed to short-term travellers, according to Dr. Alain Bouckanooge, associate vice president of clinical research and development at Sanofi’s division in Thailand. Throughout the past few years the company conducted clinical trials in tens of thousands of children in Southeast Asia and Latin America that revealed the vaccine to be 70 percent effective for those with pre-exposure to dengue and 90-95 percent effective against severe hospitalization.
Scientists have been unable to develop a vaccine for dengue in part because the virus is so complicated. It has four strains, more than other deadly diseases such as polio and smallpox. If a person gets infected with more than one type of dengue, there is a greater chance of the virus of causing hospitalization or death. Yoon said there have historically only been a few places where more than one serotype of dengue circulates at any given time, but urbanization has made it more common to have multiple serotypes in the same area.
Another challenge in testing the vaccine has been the need for expensive and time-consuming human trials. Bouckanooge says there is no good animal model that can be used as a predictor. “For dengue vaccine you don’t have that. Human dengue is quite unique.”
Even a successful vaccine won’t eliminate dengue overnight. Sanofi’s production capacity is limited, Yoon says. He estimates that the company could manufacture about 100 million doses of the vaccine annually, compared to an estimated demand of about one billion doses over five years. “So there are potentially some supply and demand issues,” he says. “Clearly there is a need for more than one vaccine and more than one vaccine manufacturer.” Dengvaxia’s side effects include systemic headaches, fatigue and light-grade fevers. No direct fatalities have been reported.
The decision whether or not to implement the vaccine will be the up to individual governments. While WHO does offer information resources to aid countries, setting up a vaccination program will provide its own set of challenges, according to Joachim Hombach, the senior advisor in WHO’s Initiative for Vaccine Research. “You need to buy the vaccine and it costs a lot money,” he says. “And you are in the business for many years — it’s essentially an open-ended commitment. You don’t want to be in a situation where you introduce a vaccine and then two years later you say, oops, sorry, we are running out of money and we have to stop this program.”
time.com